Clinicians and researchers teamed up to investigate how inappropriate proinflammatory mechanisms contribute to the pathogenesis of drug-refractory epilepsy.
Doctors treat epilepsy with anticonvulsants to control seizures, but some patients do not respond to these first-line therapies. For patients with drug-refractory epilepsy (DRE), whose seizures persist after treatment with two or more anticonvulsants, clinicians must surgically remove part of the brain tissue to cure the disease.
When first-line medicines fall short, scientists examine the molecular mechanisms of a disease to understand why and to develop alternatives. At Duke-NUS Medical School and KK Women’s and Children’s Hospital, clinicians and researchers teamed up to investigate how inappropriate proinflammatory mechanisms contribute to DRE pathogenesis. This work builds on evidence from animal models and resected brains of human patients that associated inflammation with epilepsy.2-4 Derrick Chan, a clinician scientist at KK Women’s and Children’s Hospital believes this research is an extension of his clinical work. “[T]his direction became really important, because we were looking for a less invasive way to try to help all the children with drug resistant epilepsy,” he said.
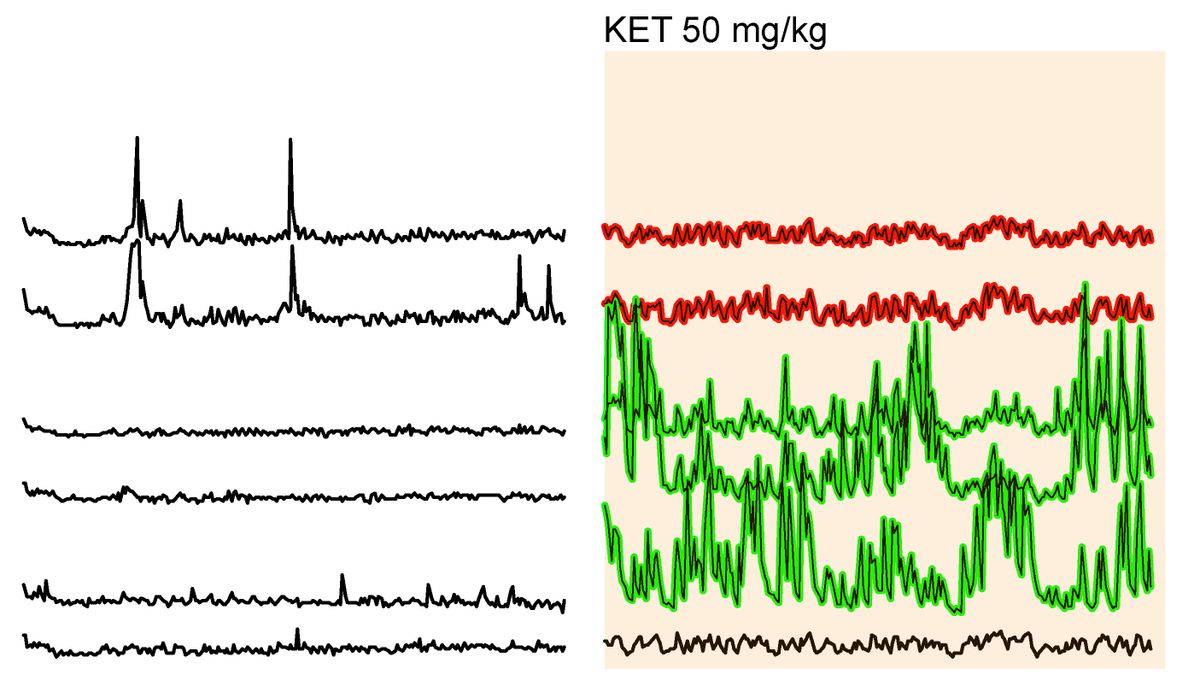
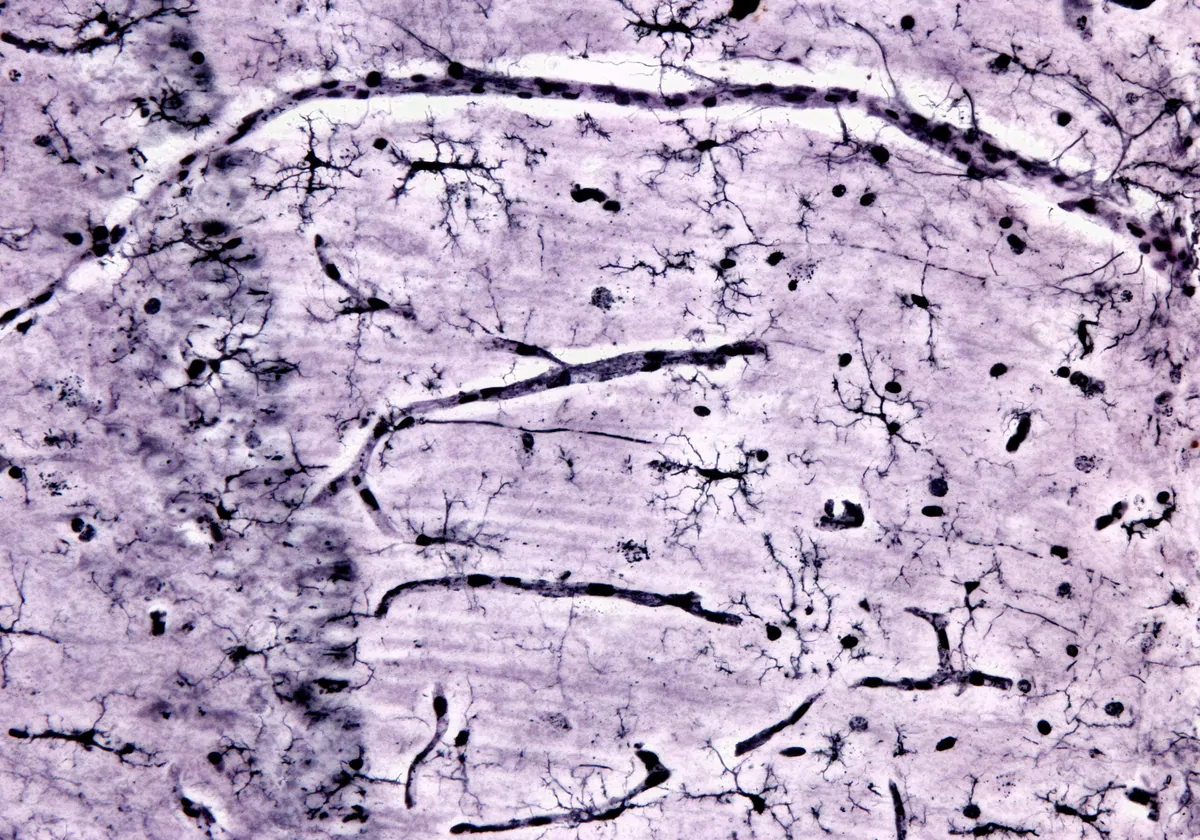
Chan and his team partnered with the immunology research group of fellow physician scientist, Salvatore Albani. In a study published in Nature Neuroscience, Chan and Albani described their efforts to understand the immunologic factors that contribute to DRE pathology.1 They examined the holistic involvement of the immune system in epileptic tissue that clinicians surgically removed from patients. The researchers used a single-cell sequencing technique called cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq), which gathers information on RNA and surface proteins in single cells.5 They uncovered a proinflammatory microenvironment in DRE lesions that resembles brain autoimmune diseases, such as multiple sclerosis (MS).
The researchers identified cell types and their functions in DRE lesions at single-cell resolution and differentiated resident brain and neurovascular cells from infiltrating immune cells. They found that the DRE microenvironment includes activated microglia and other proinflammatory immune cells, and they captured cellular interactions with additional molecular analyses. “We had not expected these interactions between microglia and other immune cells, and then how these microglia become kind of a pivot to attract all of the immune cells by starting this proinflammatory milieu inside the brain,” explained Pavanish Kumar, the first author of the study.
We were looking for a less invasive way to try to help all the children with drug resistant epilepsy.
– Derrick Chan, KK Women’s and Children’s Hospital
Previous work from Albani and Chan examined the immune patterns in the peripheral blood of patients with DRE.6 Their earlier findings underscored how systemic inflammation contributes to epilepsy pathogenesis and prompted them to investigate the local inflammatory signature in brain tissue. It can be a challenge to study brain tissue, and as Albani pointed out, access to samples is one limitation of this study. “It’s not as simple to get brain tissue from live patients… so numbers are small. That’s why we are all insisting on the word validation,” he explained. The researchers emphasized that the next steps of this work will involve animal models of epilepsy to establish a robust foundation that they will continue to build on.
“It’s a fantastic piece of science, and it’s definitely a big jump forward in our field,” said David Henshall, a professor of physiology and medical physics at Royal College of Surgeons in Ireland, who was not involved in the study. “There’s no argument from an epilepsy research side that it makes sense that there’s an immune component to this form of drug resistant epilepsy… Perhaps we can now, based on this study, design a treatment or combination of treatments that may be more precise than what has been tried so far.”
Indeed, the researchers hope to find less invasive DRE treatments, such as repurposed biologics that are safe and effective in immunological diseases. Chan also notes that epilepsy research may illuminate the role of inflammation in other neurological diseases. “I think we are in a unique position. I mean, as epileptologists, we actually have access to brain tissue.” This is in contrast to other neurological disorders like MS, for which samples that reflect early and highly active disease stages are limited.7 In this way, these findings may have effects beyond epilepsy research.
References
- P. Kumar et al., “Single-cell transcriptomics and surface epitope detection in human brain epileptic lesions identifies pro-inflammatory signaling,” Nat Neurosci, 25:956-66, 2022.
- D. Xu et al., “Peripherally derived T regulatory and γδ T cells have opposing roles in the pathogenesis of intractable pediatric epilepsy,” J Exp Med, 215:1169-86, 2018.
- K.L. van Gassen et al., “Possible role of the innate immunity in temporal lobe epilepsy,” Epilepsia, 49:1055-65, 2008.
- M. Zattoni et al., “Brain infiltration of leukocytes contributes to the pathophysiology of temporal lobe epilepsy,” J Neurosci, 31:4037-50, 2011.
- M. Stoeckius et al., “Simultaneous epitope and transcriptome measurement in single cells,” Nat Methods, 14:865-68, 2017.
- P. Kumar et al., “Proinflammatory IL-17 pathways dominate the architecture of the immunome in pediatric refractory epilepsy,” JCI Insight, 5:1-12, 2019.
- P. Vanderdonckt et al., “Tissue donations for multiple sclerosis research: current state and suggestions for improvement,” Brain Commun, 4:1-11, 2022.