Nine people with spinal injuries walked again after electrical stimulation, allowing researchers to pinpoint neurons likely underlying their recovery.
Amanda Heidt
Nov 10, 2022 | 3 min readPDF VERSION
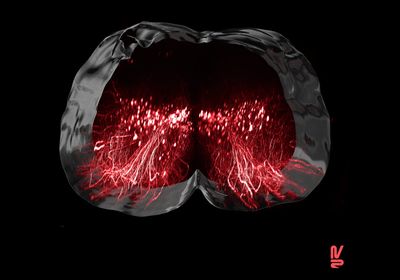
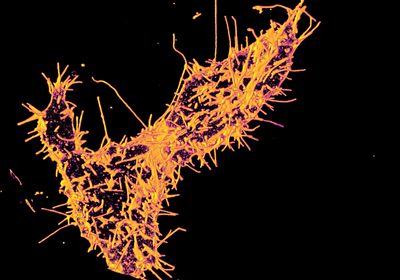
ABOVE:Visualization of the neurons involved in regaining the ability to walk after paralysisNEURORESTORE/JIMMY RAVIER
Scientists now have a better understanding of how our bodies respond to spinal cord injuries and the specific cells that may direct recovery. In a study published November 9 in Nature, researchers followed nine previously paralyzed patients undergoing a regimen of electrical stimulation who regained their ability to walk and compared their findings to mice that received a similar treatment. The team identified two neural populations that appeared to orchestrate the rewiring of connections between nerve cells following injury in the animal model. If these neurons function the same way in humans, it could lead to better-targeted therapies for spinal cord injuries.
In 2018, scientists first recognized that stimulating nerves near the site of injury—a process known as epidural electrical stimulation (EES)—could ease a person’s pain following a spinal cord injury and restore their ability to walk when combined with intensive physical therapy. But even though this method was one of few with notable clinical effects, according to experts that spoke to Science, it was never entirely clear how EES worked.
To probe the mechanistic basis underlying the therapy, Grégoire Courtine, a neuroscientist at the Swiss Federal Institute of Technology, Lausanne, who was part of the 2018 study, and his colleagues followed nine patients as they underwent a five-month regimen of EES, physical therapy, and rehab. Six had retained some feeling in their legs following their injuries, but three of the patients were entirely paralyzed from the waist down. Throughout their treatment, the team mapped the nerve cell activity in each patient.
As each person progressed and regained their ability to walk, the levels of nerve activity near the site of injury decreased. This wasn’t a surprise to the team, however. Courtine tells Nature that it mirrors what happens in the brain during learning. “When you learn a task, that’s exactly what you see—there are less and less neurons activated as you get better at it,” he says. And speaking to New Scientist, coauthor Jocelyne Bloch of the University of Lausanne notes that following a spinal cord injury, there is a lot of “chaotic activity” as the body responds. The rehabilitation process “organises the network of cells and you actually increase the activity of a specific type of cell, while all the other cells are not activated.”
Together, these findings suggested to the team that there may be a subset of neurons responsible for coordinating a person’s recovery. The scientists decided to probe this idea in mice, inducing spinal cord injuries in the animals and mimicking the recovery process to try and recapitulate their findings. When they applied a murine version of EES to mice with paralyzed rear legs, they saw the same dampening of activity that they had seen in humans.
The researchers next measured gene expression across populations of neurons in mouse spinal tissue, categorizing the neurons into subsets based on their expression profiles. Using a machine learning algorithm, the team was able to map changes in gene expression that corresponded to stages in the recovery trajectory of the human patients.
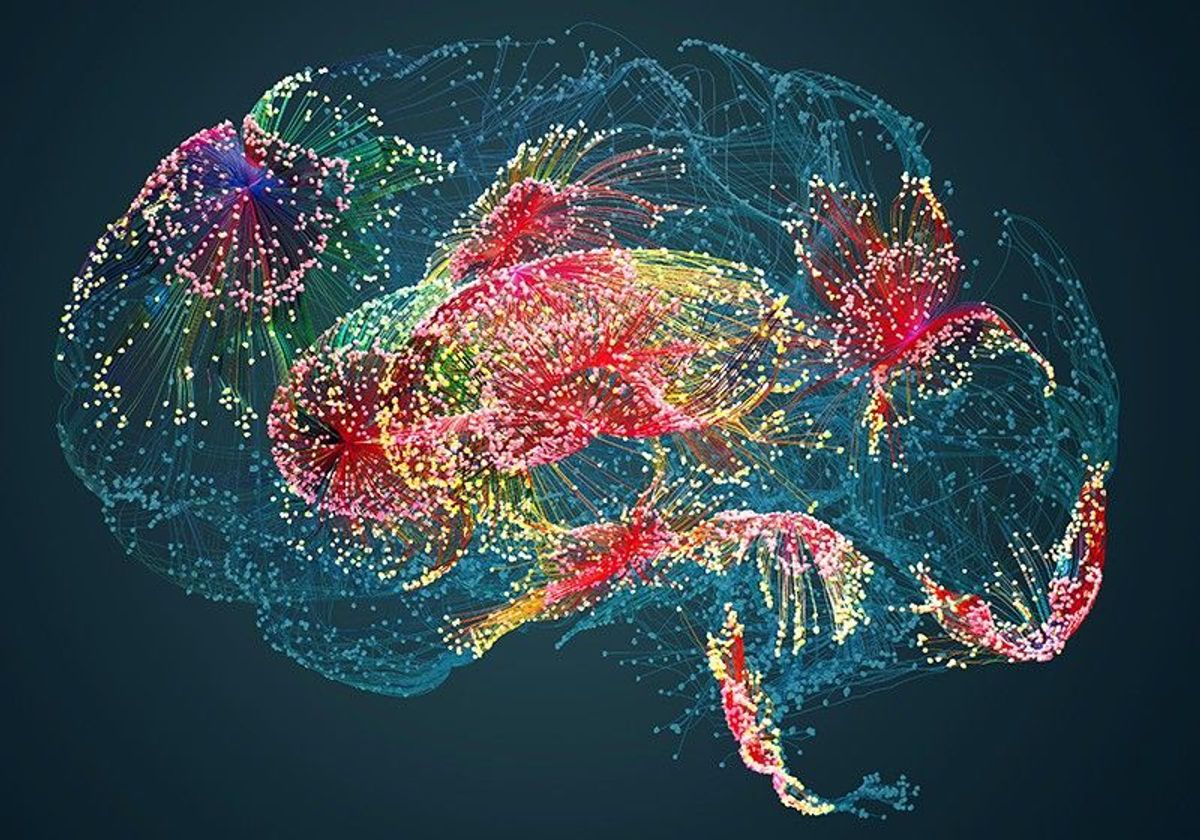
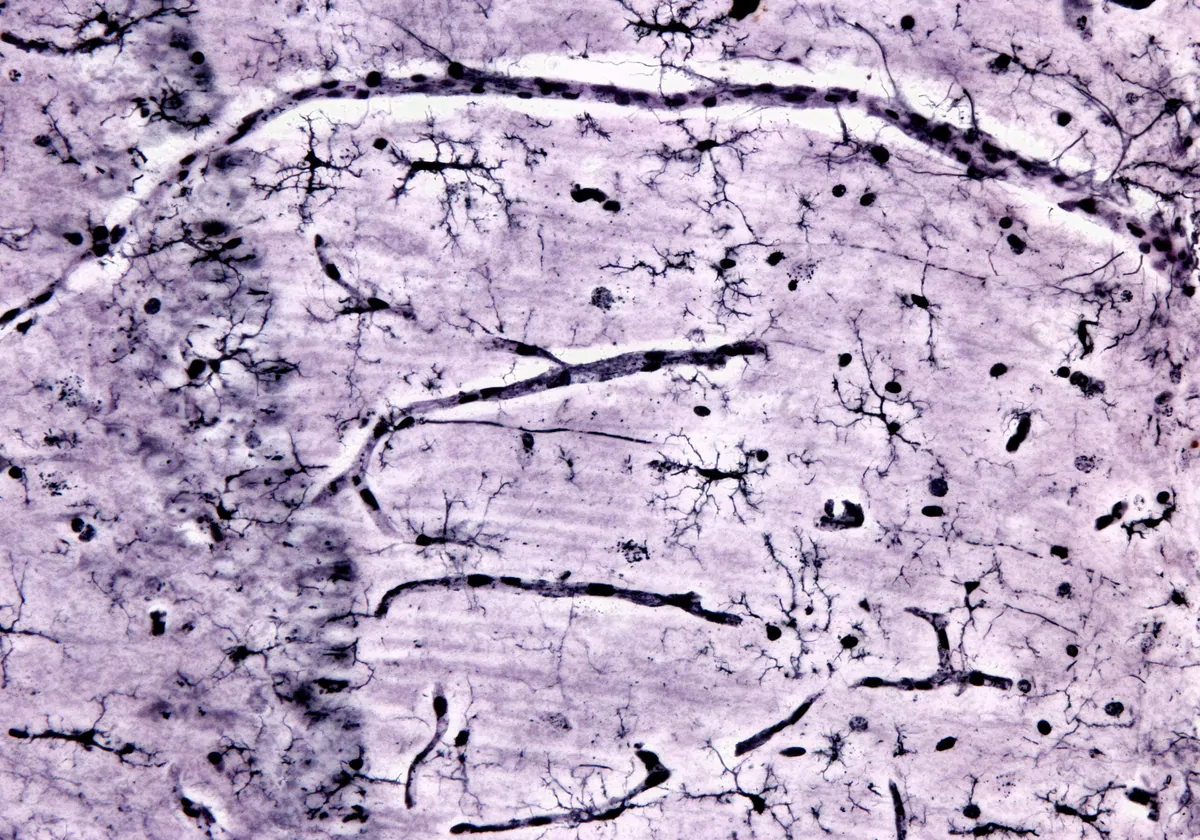
Scientists identified 36 distinct neuronal subpopulations based on gene expression profiles and mapped their distribution across the lower spinal cord.
NEURORESTORE/JIMMY RAVIER
Specifically, the algorithm identified two subpopulations of excitatory interneurons—nerve cells that connect motor and sensory neurons—that expressed the genetic markers Vsx2 and Hoxa10 as potential drivers of the animals’ recovery. When the scientists applied optogenetics to inactivate these cells, the mice lost their ability to walk again, regaining it only when the cells were activated. In mice without a spinal cord injury, silencing the cells did nothing, suggesting that such cells only become essential to movement following an injury, Science reports.
Speaking to New Scientist, Marc Ruitenberg, a neurologist at the University of Queensland who studies spinal cord injury and was not involved in the study, says that the hope these findings give to those with spinal injuries “is incredible.”
Other researchers have a more cautious interpretation. Eiman Azim, a neuroscientist at the Salk Institute for Biological Studies in California, tells Nature that we don’t yet have technologies capable of manipulating human neurons in the same ways as mouse neurons, making it currently impossible to collect direct evidence that EES induces the same changes in humans. He says he suspects, though, that the same neurons are probably responsible for the effect the team saw in their patients because spinal architecture is very similar across vertebrates.
Courtine has since launched a start-up called ONWARD to commercialize the technology and plans to recruit patients in the US for a new trial in 2024, Science reports. Some members of the group, including Courtine, previously used EES to restore arm movement and hand grip in nonhuman primates, and Courtine tells Science he’s interested in seeing whether EES can restore other functions that are lost to injury, such as bladder and bowel control, or the ability to have sex.