After injecting moderate doses of the dissociative anesthetic into the animals, previously “awake” brain cells go dark, and those that had been dormant suddenly light up.
ABOVE:© ISTOCK.COM, ANDREUS
In the 1950s, scientists on a mission to create better anesthesia drugs synthesized phencyclidine, commonly known as PCP. Though PCP worked well to keep most people unconscious during surgical procedures, some experienced what the authors of a 1959 trial described as “delirium and hallucinations which, although usually of a highly pleasurable nature, are sometimes rather terrifying to the patients.” This so-called dissociated state—when what the brain experiences is disconnected from reality—lasted as long as 12 hours.
Seeking a shorter-acting agent, researchers in the 1960s made a compound that’s structurally related to PCP called ketamine. Ketamine remains a common anesthetic today, says Joe Cichon, a neuroscientist and anesthesiologist at the University of Pennsylvania’s Perelman School of Medicine. At lower doses than would be used for anesthesia, people remain conscious yet experience a similar dissociated state as with PCP but for far less time. In the 2000s, researchers found that these lower, so-called subhypnotic doses of ketamine have an antidepressant effect that can last for several weeks, well after the body has metabolized the drug, Cichon says. “And still to this day, after 60 years of being available for human use . . . we still don’t quite understand how it exerts all these different effects over a wide range of doses—that’s the mystery of ketamine.”
Now, Cichon and his colleagues have uncovered a new clue: By using calcium photon imaging, they found that the drug flips a “switch” in mice brains, shutting off neurons that had been firing in the awake state while activating a separate group of previously dormant neurons. The findings, published on November 24 in Nature Neuroscience, suggest this activity switching could result from suppressing neurotransmission via one set of receptors in certain cells while promoting neurotransmission via a different set of receptors in others.
“[This study] allows us to get a view of what’s happening in the brain during this dissociated state, which we did not have a very good view of microscopically,” says Alex Kwan, a neuroscientist at Cornell University’s Meinig School of Biomedical Engineering, who was not involved in the research. Kwan adds that the researchers investigated the switching effect in “many brain areas and many layers to confirm their findings, and it seems to be quite widespread.”
Flipping neurons on and off
To probe how ketamine changes neural activity, the researchers gave the drug to transgenic mice and measured how it impacted cells in multiple layers of their neocortex. This region is the six-layered, outer surface of the mammalian brain that’s responsible for higher functions such as remembering, thinking, learning, and reasoning, explains study coauthor and UPenn neuroscientist and anesthesiologist Alex Proekt. The mice were modified such that specific excitatory neurons released calcium when firing, which the researchers recorded with their imaging technique.
See “CRACK Method Reveals Novel Neuron Type in Mouse Brain”
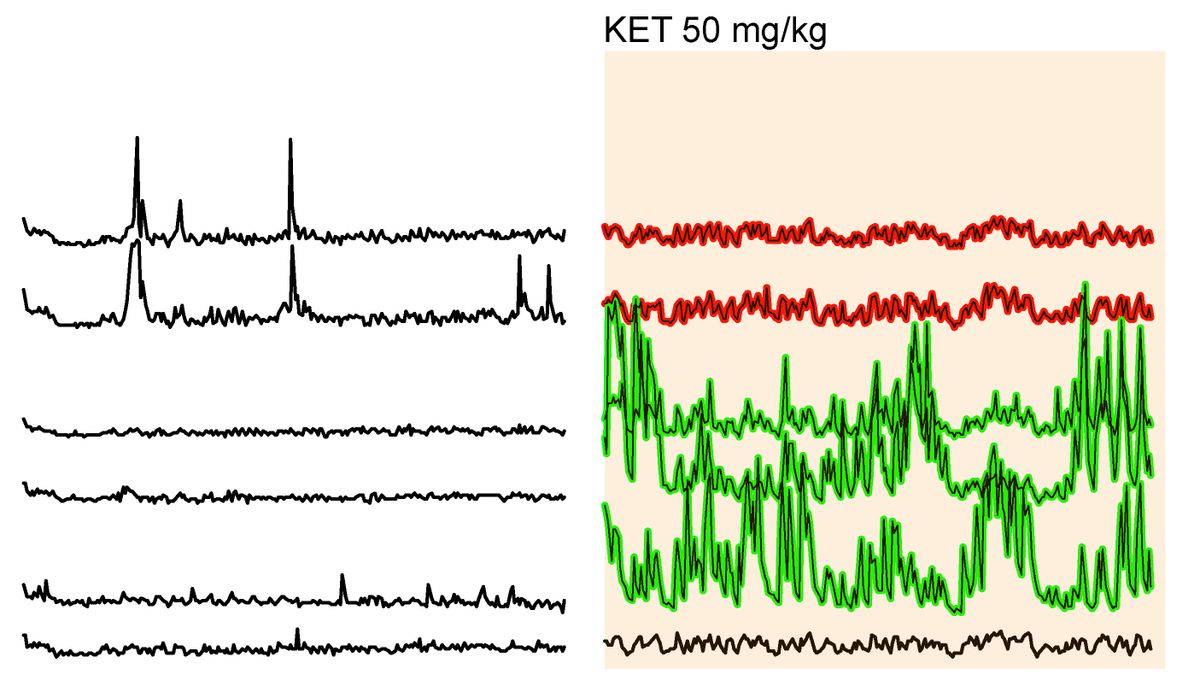
“The mouse is a perfect specimen to do this type of study because the skull and the brain itself [are] very flat,” unlike the human brain’s tortuous nooks and crannies, says Cichon. Before injecting the mice with ketamine, the calcium signal from certain neurons fluctuated, indicating they were firing during the awake-state, while the signal from dormant neurons were essentially flat-lined. About 10 to 20 minutes after they injected subhypnotic doses of ketamine into the mice’s abdomens, some neurons appeared to switch off while others suddenly came to life.
The activity of six murine cortical neurons recorded during normal wakefulness (left) compared to the same six cells in the presence of subhypnotic doses of ketamine (right), which shows increased activity in three cells (green), decreased activity in two (red), and no change in one (black).
CICHON ET AL; NATURE NEUROSCIENCE
“Ketamine—in a way that was previously not known—induces a complete switch in active circuits,” says Cichon. “So you’re trading off one active circuit for a new circuit that emerges basically from darkness.”
The researchers repeated the experiment, but this time applying the drug directly onto each mouse’s neocortex. Because they saw a similar switching effect, the researchers concluded that whatever mechanism was at play occurred at the neuronal level, not somewhere upstream, as could have been the case when injecting the drug into the mice’s abdomens. In addition, Cichon and colleagues repeated the experiments in different regions as well as in deeper layers of the cortex, which again exhibited a similar swap in cellular activity.
“I don’t think I know any other drug that would do such a thing,” says Kwan. “So to observe that from this study across multiple [neocortex] areas across multiple layers of neurons—it’s just a very surprising finding,” and one that he says he intends to look for in his own data.
Information freeways versus frontage roads
Cichon and his colleagues hypothesized that ketamine deactivated certain neurons by blocking their N-methyl-D-aspartate (NMDA) receptor, which is something the drug was already known to do. To probe into the mystery of why different neurons flipped on, the researchers turned to other known ketamine effects: specifically, the drug’s ability to suppress cells that inhibit excitatory neurons called interneurons, and to block hyperpolarization-activated cyclic nucleotide-gated (HCN) channels—transmembrane proteins that regulate the excitability of neurons.
When the researchers injected the mice with ketamine as well as compounds that blunt the drug’s normal suppression of two types of interneurons—essentially forcing these cells to remain active—the activity switch didn’t occur, indicating that the switching effect depends on ketamine’s ability to subdue them. In another experiment, the researchers injected different compounds that blocked HCN channels—mimicking the effect of ketamine without actually injecting it. This induced a similar switching effect, suggesting these channels also play a role.
So it’s not that ketamine itself treats depression. It’s [that] ketamine produces some state of the brain that then leads to changes in the brain.
—Alex Proekt, University of Pennsylvania
Considering the combined results, a possible explanation for how NMDA receptors, interneurons, and HCN channels contribute to the neuronal flip-flop emerged from a neuroscience adage: “Neurons that fire together, wire together,” says Proekt. Neurons that fire synchronously tend to rely on NMDA-neurotransmission, and the synaptic connections between these cells strengthen the more they’re used, he says. Like a muscle bulking up with repeated weight lifting, these pathways may become so strong that they end up functioning like information freeways, conducting most of the cortex’s traffic. On the other hand, neurons that fire asynchronously may have atrophied synaptic connections connecting them, says Proekt, which may function as hardly- or never-traveled frontage roads.
With ketamine barricading the NMDA highways by hindering interneurons’ ability to inhibit excitation and by impeding HCN channels’ capacity to regulate excitation, the drug could create a scenario where the traffic from the freeway gets diverted to the less-traveled side roads, stimulating these neurons into action. Though NMDA receptors on these newly awakened neurons would remain suppressed, the cells could still transmit signals via AMPA receptors.
Kwan calls the team’s calcium imaging technique “state of the art” but relatively slow method for measuring brain activity—on the order of seconds. “I think a very good next step would be to use electrophysiology to record electrical brain activity, which will give you millisecond timescale resolution,” he says, adding that this approach could reveal finer detail on how activity switching occurs. Kwan also notes the researchers’ method is indirect. Because NMDA receptors alter the flow of calcium into neurons’ dendrites, the team’s imaging technique may have, at least in part, been picking up on the action of ketamine on the receptors rather than neuronal activity. Using electrophysiology would directly measure voltage, but he concedes it would also make it more difficult to measure individual cells, as the researchers did in this study.
Proekt points out that the researchers’ mechanistic theory remains speculation, but their findings bring scientists closer to understanding the unusual ketamine-induced brain state that helps treat depression, and possibly other conditions such as addiction.
Opening new paths to treating depression
At subhypnotic levels, ketamine leaves a person’s system in about one or two hours, but the antidepressant benefits last for several weeks, says Proekt. “So it’s not that ketamine itself treats depression,” he argues. “It’s [that] ketamine produces some state of the brain that then leads to changes in the brain.” He and his colleagues plan to further explore how the newly discovered activity switch plays into this neuroplasticity, Proekt says, adding that the research could lead to new depression treatments.
James Murrough, a psychiatrist who was not involved in the work but who studies the antidepressant effects of ketamine at Mount Sinai’s Icahn School of Medicine points out that using mice in this analysis presents an important limitation when it comes to gleaning insights about humans. “The problem we often have in [psychiatric disorders], which are fundamentally disorders of feeling and thinking, [is that] those cannot be measured in an animal,” he says. Instead, researchers rely on behavioral surrogates, such as a characteristic head-twitching that’s indicative of the dissociative state in mice. In reality, “we don’t know for sure what these mice are experiencing or if they’re dissociated.”
Nonetheless, he says, these researchers are “really finding novel evidence of—at the cellular level—what ketamine is doing in the brain.” The neuron switching effect shown in this paper represents an important piece of the puzzle to treating clinical depression, which he likens to a state of clogged thinking patterns that cause persistent negative thinking. Ketamine seems to function as a reboot for the mind, he says, similar to how restarting a frozen computer can unlock it.
“We need the fingerprint of what [the drug] does in the brain for fundamental novel drug discovery for depression,” says Murrough. “I think that this [research] might move us a little closer in that direction.”